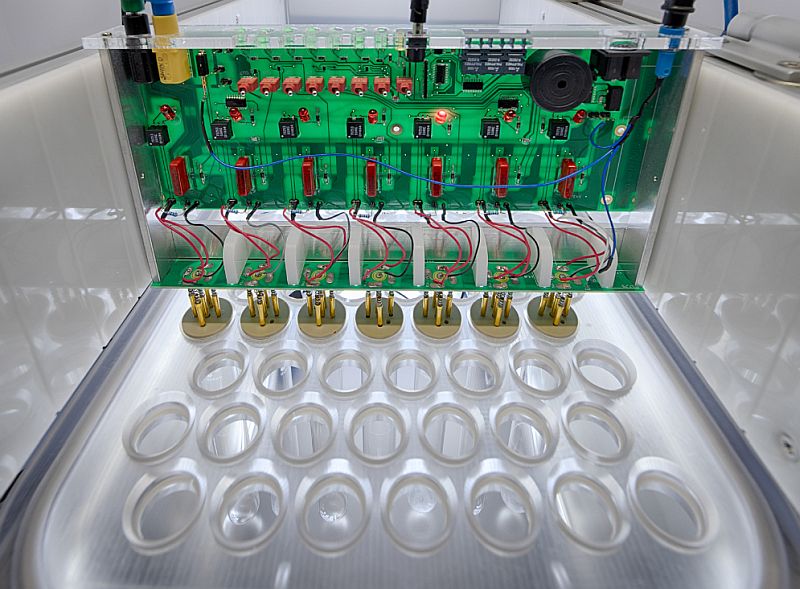
Characterisation and reliability testing of implantable devices
The long-term reliability and characterization of implantable biomedical devices is critical to their successful translation into clinical use. Implantable devices must withstand the harsh biological environment and minimize the effects of foreign body reactions. To avoid implant failure, reliable encapsulation is critical. The reliability and performance of the implant are also strongly influenced by its electrodes. Detailed characterization of the encapsulation and implantable electrodes is essential for comparison with state-of-the-art technologies. To better quantify and analyze the performance of implantable devices, the Technologies for Bioelectronics group offers various measurement methods including:
- Automated test setups for long term testing
- Soak tests
- Accelerated aging tests
- Large-scale/Batch testing
- Electrochemical analysis techniques
- Electrical impedance measurements
- Voltage transient measurements
- Cyclic voltammetry
These methods can be adapted to accommodate different test samples, so feel free to contact us to discuss your specific requirements in more detail.
 Fraunhofer Institute for Reliability and Microintegration IZM
Fraunhofer Institute for Reliability and Microintegration IZM